World Health Organisation (WHO) said it had prequalified the second dengue vaccine, TAK-003, in the effort against mosquito-borne illness prevalent in tropical and subtropical regions.
The new vaccine for dengue received prequalification from WHO on May 10, 2024. TAK-003 is the second dengue vaccine to be prequalified by WHO.
According to WHO TAK-003, developed by Takeda, is a live-attenuated vaccine containing weakened versions of the four serotypes of the virus that cause dengue.
The health body recommended the use of TAK-003 in children aged 6–16 years in settings with high dengue burden and transmission intensity, adding that vaccine should be administered in a two-dose schedule with a three-month interval between doses.
WHO’s Director for Regulation and Prequalification, Dr Rogerio Gaspar, said: “The prequalification of TAK-003 is an important step in the expansion of global access to dengue vaccines, as it is now eligible for procurement by UN agencies including UNICEF and PAHO. With only two dengue vaccines to date prequalified, we look forward to more vaccine developers coming forward for assessment, so that we can ensure vaccines reach all communities who need it.”
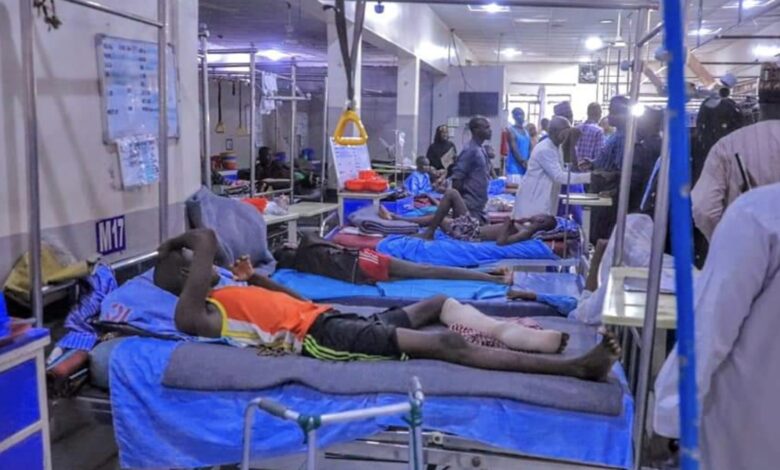
Dengue is a vector-borne disease transmitted by the bite of an infected mosquito. Severe dengue is a potentially lethal complication which can develop from dengue infections.
“It is estimated that there are over 100-400 million cases of dengue worldwide each year and 3.8 billion people living in dengue-endemic countries, most of which are in Asia, Africa, and the Americas. The largest number of dengue cases reported was in 2023 with the WHO Region of the Americas reporting 4.5 million cases and 2,300 deaths. Dengue cases are likely to increase and expand geographically due to climate change and urbanisation.”
The prequalification of TAK-003 comes alongside the existing WHO-approved CYD-TDV vaccine developed by Sanofi Pasteur.